Dear Readers,
This week’s blog is about the Cold chain logistics’ role in ensuring effective vaccine storage, handling, stock management, rigorous temperature control in the cold chain, and maintenance of adequate logistics management and information systems.
Ambimat Electronics, with its experience of over four decades as an ODM of IoT products, wishes to draw the attention of its customers and readers of blog posts towards Cold Chain Logistics.
Inbound logistics and distribution as major supply-chain pain points
The height of the first wave of COVID-19 infections revealed several logistics-related challenges in two links of the supply chain – inbound logistics and distribution. Particularly as related to personal protective equipment (PPE), product-quality issues, constrained transportation capacity, complex customs processes, and regulations increasing the risk of delays, warehousing challenges, and limited transparency regarding stock levels all posed significant problems. By partnering with a suitable logistics service provider, governments can alleviate these challenges.
Transporting temperature-sensitive products, from bananas to vaccines, is inherently risky. The improper temperature during shipment can spoil food and render medicine ineffective, leading to consumer safety issues. Rejecting mishandled shipments carries an enormous economic cost. Supply chain experts estimate that a single shipment by refrigerated truck (or “reefer”) can be worth up to $50 million in the case of pharmaceuticals, compared to $100,000 for standard container loads.
Properly managing the cold chain—the processes and technologies used to transport temperature-sensitive products from origin to destination—is a massive logistical effort. Drivers and dispatchers need to coordinate pickups and deliveries; equipment maintainers need to be alerted to potential problems before shipments are endangered; operations managers need to access detailed auditing information to ensure compliance with regulations and guarantee shipments can be trusted. All of this requires coordination and expertise, and carries a commensurate cost: according to industry forecasts by Pharmaceutical Commerce, cold chain logistics represents a $15 billion market in the biopharma sector alone.
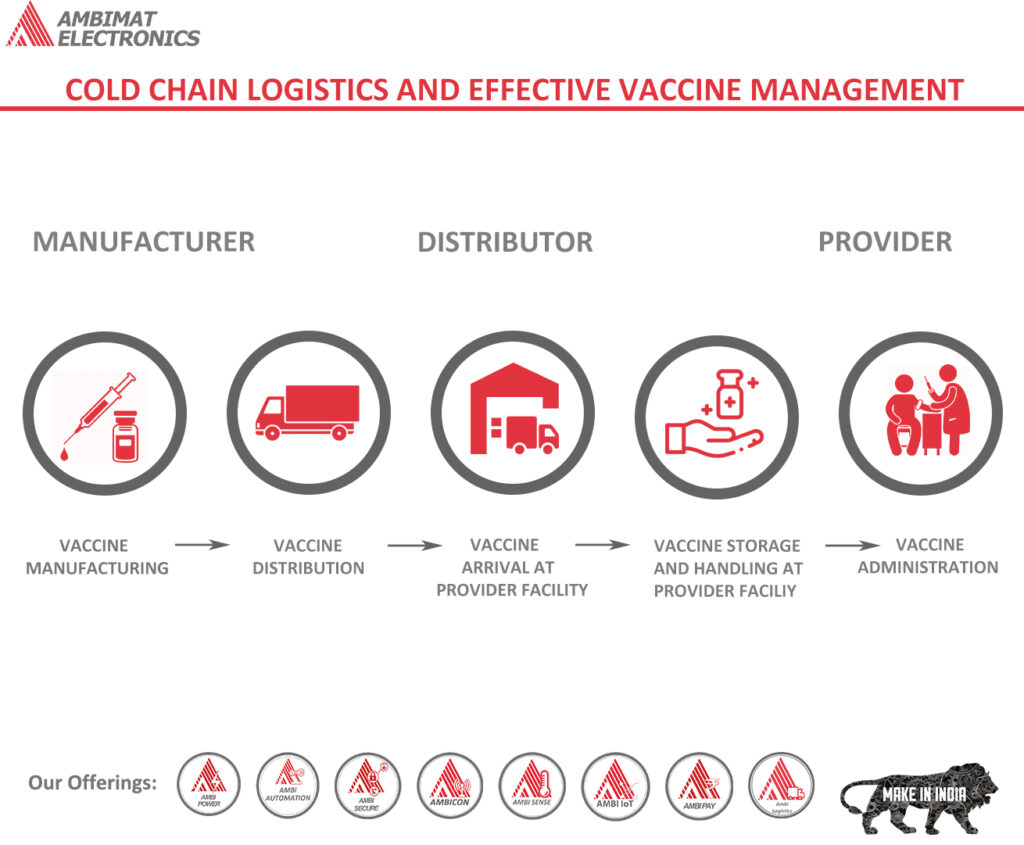
Vaccine distribution as the next logistics hurdle
Currently, there are some 250 candidates for a COVID-19 vaccine in various stages of development. The diversity and novelty of these potential vaccines – and the unprecedented speed at which they are being developed – raises multiple questions from a logistics perspective. Specifically, the potential vaccines are being developed on multiple platforms with each platform generating the immune response through a different mechanism. Four out of the six vaccine frontrunners, for example, are based on rather new or even experimental platforms, while two are based on traditional platforms. Different platforms will likely come with different temperature requirements for transportation and storage. As a result, regional distribution capabilities, as well as packaging and transportation sustainability, will all likely be a function of whether temperature requirements for safe and efficacious vaccines will be as low as -80°C or end up falling in the +2–8°C range.
Proper monitoring is essential for drugs and medical supplies in the pharmaceutical industry, as they are often limited in quantity and need to reach their destinations in a specific amount of time. Effective Vaccine Management (EVM), launched by the World Health Organization (WHO) and UNICEF which is a system designed to identify the strengths and identify best-practices in vaccine(cold chain) logistics.
Vaccine arrival:
All pre-shipment and arrival procedures ensure that every shipment of vaccines from a manufacturer reaches its first destination in a country (a primary vaccine store or central medical store) in a satisfactory condition (no breaks in the cold chain nor damaged vaccines).
Temperature control:
All vaccines and their diluents are stored and distributed within a cold-chain system that maintains, at all times, the WHO-recommended temperatures ranges for all types of vaccines.
Storage capacity:
The national supply chain system has sufficient and quality cold storage, dry storage, and transport storage capacity to accommodate all vaccines, diluents, and injection supplies needed for the national immunization program.
Infrastructure:
The status and the layout of storage buildings, cold-chain equipment, and vehicles enable the supply chain system to function effectively.
Maintenance:
Preventive and curative maintenance systems are standard and operational for storage buildings, cold-chain equipment, and vehicles used to distribute vaccines.
Stock management:
Systems and procedures for managing the stocks of vaccines are effective in vaccine handling, physical inventory, stock-control systems, adequate stock-level policy, good warehousing practice, and disposal procedures for damaged and expired vaccines.
Distribution:
The transport of vaccines between each level in the supply chain is effective, including the correct use of passive containers (cold boxes), packing practices with coolant packs (conditioned ice-packs or cool water packs), temperature indicators, and maintaining transport contingency plans.
Our Cold Chain Logistics solutions leverage IoT to monitor the location, condition, and integrity of perishable shipments from points of production to consumption. The solution addresses challenges with respect to Temperature Monitoring, Shipment Tracking, and Reduction in Spoilage of Goods. Additionally, the solution creates a transparent and seamless flow of shipment ownership information. All of these lead to a reduction in shipment costs & damages and improved transactional efficiencies resulting in an improved customer experience.
About Ambimat Electronics:
With design experience of close to 4 decades of excellence, world-class talent, and innovative breakthroughs, Ambimat Electronics is a single-stop solution enabler to Leading PSUs, private sector companies, and start-ups to deliver design capabilities and develop manufacturing capabilities in various industries and markets. AmbiIoT design services have helped develop Smartwatches, Smart homes, Medicals, Robotics, Retail, Pubs and brewery, Security.
Ambimat Electronics has come a long way to become one of India’s leading IoT(Internet of things) product designers and manufacturers today. We present below some of our solutions that can be implemented and parameterized according to specific business needs. AmbiPay, AmbiPower, AmbiCon, AmbiSecure, AmbiSense, AmbiAutomation.
To know more about us or what Ambimat does, we invite you to follow us on LinkedIn or visit our website.
References:-
https://pharmaceuticalcommerce.com/clinical-operations/the-2018-market-for-pharma-cold-chain-logistics-is-15-billion/
https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/National_EVM_Assessment_Report_2018.pdf
https://www.unicef.org/rosa/media/1281/fil/WHO%20UNICEF%20statement.pdf
https://www.who.int/immunization/programmes_systems/supply_chain/evm/en/